Lutetium-177 PSMA Therapy for Prostate Cancer
Home » Lutetium-177 PSMA Therapy
- Experience a whole new world of medicine - Theranostics - at the state of the art facility
- Combine your therapy with imaging for personalized, targeted treatment
- Clean, comfortable and premium isolation rooms which are regularly sanitized.
- Pioneers of theranostics in India
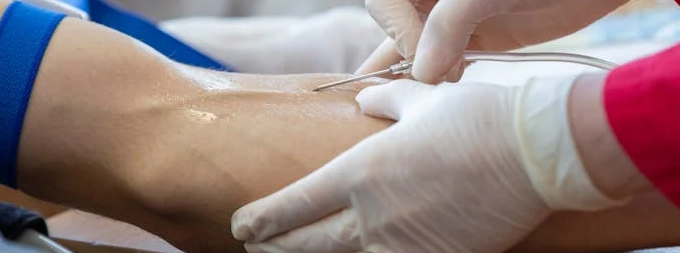
What is Lutetium-177 PSMA Therapy?
Who is it For?
- Metastatic Castration-Resistant Prostate Cancer (mCRPC): This is advanced prostate cancer that has spread to other parts of the body and no longer responds to hormone therapy aimed at reducing testosterone levels.
- Recurrent prostate cancer : Men who have undergone other treatments like surgery or chemotherapy for prostate cancer but the cancer has recurred.
- PSMA Positive Scans: A PSMA PET scan is often used to confirm the presence of PSMA-positive cancer cells before considering Lutetium-177 PSMA therapy.
How Does it Works?
- PSMA Targeting: Prostate cancer cells often have high levels of a protein called prostate specific membrane antigen (PSMA) on their surface. Lutetium-177 PSMA therapy leverages this by using a radioactive isotope, Lutetium-177 (Lu-177), attached to a molecule that specifically binds to PSMA.
- Targeted Radiation Delivery: Once the Lu-177 PSMA complex reaches the bloodstream, it travels and binds to PSMA on prostate cancer cells. The Lu-177 then emits short range beta radiation, effectively damaging and destroying the targeted cancer cells.
- Minimized Side Effects:Because the radiation is concentrated on PSMA- positive cells, there s less damage to healthy tissues compared to traditional radiation therapy techniques.
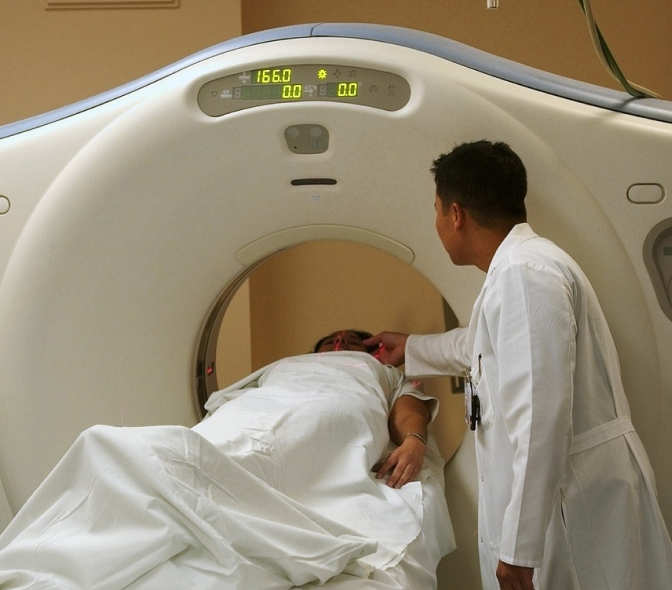
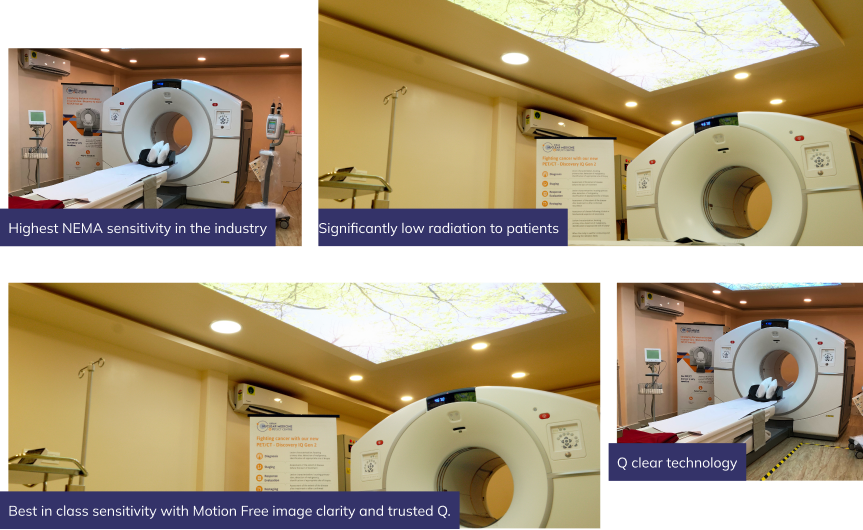
The Next Generation Technology is Here at Kiran PET CT - GE-DISCOVERY IQ GEN 2
Why Choose Us
Pioneering Theranostics
At Kiran Nuclear Medicine and PET CT Center, we're leading the way in theranostics, integrating advanced diagnostics with personalized treatment plans.

Precision and Effectiveness
Our commitment to theranostics means that you receive precise diagnoses and targeted therapies, leading to more effective treatment outcomes. We leverage cutting-edge technology and expertise to deliver the highest standard of care.

Personalized Medicine
We prioritize your individual health needs, providing customized treatment plans that consider your medical history, preferences, and treatment goals.
Meet Our Doctors
Frequently asked questions
Lutetium-177 PSMA Therapy is a targeted radionuclide therapy used in the treatment of metastatic castration-resistant prostate cancer (mCRPC). It involves the use of a radioactive isotope, Lutetium-177, coupled with a PSMA (Prostate-Specific Membrane Antigen) targeting molecule. This combination allows for the selective delivery of radiation to prostate cancer cells that express PSMA.
PSMA is a protein that is overexpressed on the surface of prostate cancer cells. Lutetium-177, a beta-emitting radionuclide, is attached to a molecule that specifically binds to PSMA. When injected into the patient’s bloodstream, the PSMA-targeted molecule seeks out and binds to prostate cancer cells wherever they may be in the body. The radiation emitted by Lutetium-177 then damages the cancer cells, leading to their destruction.
Lutetium-177 PSMA Therapy is typically recommended for patients with metastatic castration-resistant prostate cancer (mCRPC) that has progressed despite standard treatments such as hormone therapy and chemotherapy. It may also be considered for patients who are not candidates for other treatments or who have experienced disease progression after previous therapies.
Lutetium-177 PSMA Therapy is generally considered safe when administered by trained healthcare professionals in specialized nuclear medicine facilities. However, like any medical treatment, there are potential side effects and risks associated with the therapy, including bone marrow suppression, kidney damage, and radiation exposure to other tissues. Patients will be closely monitored during and after treatment to manage any adverse effects.
Before undergoing Lutetium-177 PSMA Therapy, patients may undergo imaging studies such as PET/CT scans with PSMA tracers to assess the extent of disease and PSMA expression. The therapy itself involves the intravenous administration of Lutetium-177 PSMA, typically as a series of treatments spaced several weeks apart. Patients may experience side effects such as fatigue, nausea, and dry mouth, which are usually temporary and manageable.
Lutetium-177 PSMA Therapy has shown promising results in clinical studies, with many patients experiencing a reduction in tumor size, relief of symptoms, and improved quality of life. However, individual responses to the therapy can vary, and not all patients may derive the same benefit. The therapy may also be used in combination with other treatments as part of a comprehensive management plan for metastatic prostate cancer.




