Ever Heard Of Therapy+Diagnostics?
Home » Theranostics Therapy
- Experience a whole new world of medicine - Theranostics - at the state of the art facility.
- Combine your therapy with imaging for personalized, targeted treatment
- Clean, comfortable and premium isolation rooms which are regularly sanitized.
- Pioneers of theranostics in India
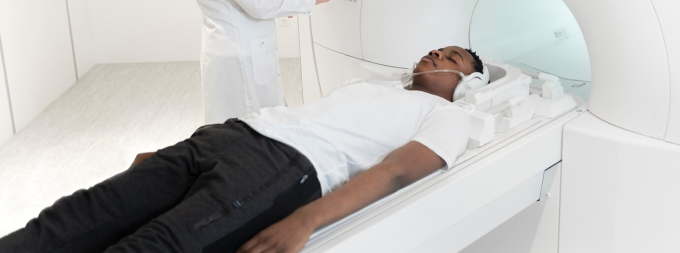
What Is Theranostics ?
Applications Of Theranostics
- Oncology: Personalized cancer treatment through targeted therapy and imaging.
- Endocrinology: Localization of hormone secreting tumors and targeted therapy.
- Neurology:Early diagnosis and tailored treatment for neurological disorders.
- Cardiology: Precise detection and management of cardiovascular diseases.
- Radiology and Nuclear Medicine: Dual purpose radiopharmaceuticals for imaging and therapy.
- Immunology and Rheumatology: Guided immunomodulatory therapy for autoimmune disorders.
Diagnose And Treat These Cancers With Our Theranostic Facility:

I131 RADIO-IODINE THERAPY for thyroid cancer
Lu 177 Dotanoc therapy for neuroendocrine tumors
Lu 177 PSMA therapy for neuroendocrine tumors
Lu 177 FAPI therapy for overy cancer,Hepatobiliary etc.
How Does Theranostics Work? Theranostics Typically Involves The Following Steps:
- Diagnosis: Advanced imaging techniques, such as positron emission tomography (PET), single photon emission computed tomography (SPECT), or molecular imaging, are used to identify biomarkers or molecular targets associated with the disease.
- Target Identification: Biomarkers identified through diagnostic imaging help pinpoint specific molecular targets or pathways implicated in the disease process.
- Treatment Selection: Based on the molecular profile of the disease, targeted therapies, such as monoclonal antibodies, small molecule inhibitors, or radioisotope therapies, are selected to precisely target the identified molecular pathways.
- Therapeutic Monitoring: Diagnostic imaging techniques are used to monitor the response to treatment, allowing for real time adjustments and optimization of therapy.
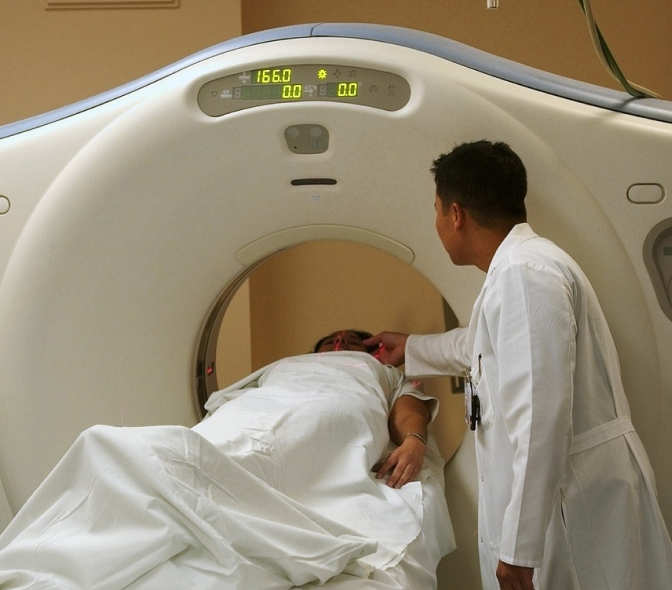
The Next Generation Technology is Here at Kiran PET CT - GE-DISCOVERY IQ GEN 2
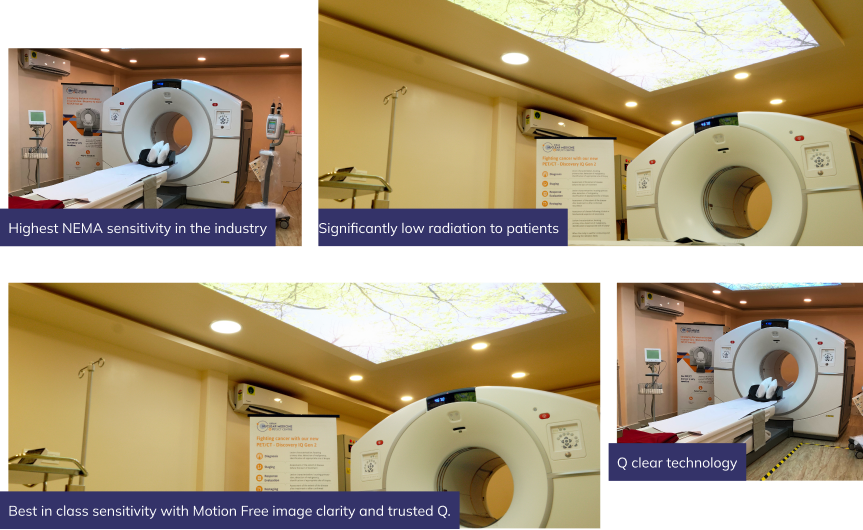
Why Choose Us
Pioneering Theranostics
At Kiran Nuclear Medicine and PET CT Center, we're leading the way in theranostics, integrating advanced diagnostics with personalized treatment plans.

Precision and Effectiveness
Our commitment to theranostics means that you receive precise diagnoses and targeted therapies, leading to more effective treatment outcomes. We leverage cutting-edge technology and expertise to deliver the highest standard of care.
Personalized Medicine
We prioritize your individual health needs, providing customized treatment plans that consider your medical history, preferences, and treatment goals.
Meet Our Doctors
Frequently Asked Questions
Theranostic Therapy is an approach that combines therapeutic and diagnostic capabilities into a single agent or procedure. It involves the use of diagnostic tools to identify specific molecular targets in a patient’s body, followed by the administration of a therapeutic agent that targets the same molecular pathway. This integrated approach allows for personalized treatment based on individual patient characteristics.
Theranostic Therapy typically begins with diagnostic imaging techniques such as PET (Positron Emission Tomography) or SPECT (Single Photon Emission Computed Tomography) scans, which use radioactive tracers to identify specific biomarkers or molecular targets in the body. Once the target is identified, a therapeutic agent, such as a radioactive isotope or a targeted drug, is administered to selectively treat the diseased tissue while sparing healthy cells.
Theranostic Therapy offers several advantages, including personalized treatment tailored to the individual patient’s molecular profile, enhanced targeting of diseased tissue while minimizing damage to healthy cells, and real-time monitoring of treatment response using diagnostic imaging. This approach can improve treatment outcomes, reduce side effects, and optimize the use of healthcare resources.
Theranostic Therapy has applications in various medical fields, including oncology, neurology, cardiology, and endocrinology. It is commonly used in cancer treatment to target tumors with high precision, but it can also be employed in the management of other conditions such as neuroendocrine tumors, thyroid disorders, and certain types of cardiovascular disease.
Theranostic Therapy is generally considered safe when performed by trained healthcare professionals in appropriate clinical settings. However, like any medical intervention, there are potential risks and side effects associated with specific therapeutic agents or procedures. Patients undergoing Theranostic Therapy will be closely monitored for adverse reactions, and their treatment plan will be adjusted as needed to ensure safety and efficacy.
While Theranostic Therapy is a rapidly evolving field with promising potential, it may not yet be widely available in all healthcare settings. Availability can vary depending on factors such as geographic location, healthcare infrastructure, and regulatory approval of specific therapeutic agents or procedures. However, as research and technology continue to advance, the accessibility of Theranostic Therapy is expected to increase over time.



